Microfluidics and individual cell handling
Microfluidics technology is being developed and incorporated into the Cell Retainers, to enable an effective use of extremely small sample volumes, precise cell treatment in the CR, and handling, separating and transferring single (rare) cells. In order to achieve these aims, the Cell Retainers have been modified to include internal fluid cross-vessels, reservoirs and input-output ports. This system is controlled by external micro-pumps, governed by automation software. The selection, segregation and re-location of specific cells is performed, within the internal microfluidic system, by laser tweezers and capillary micromanipulation.
Expanding CR capabilities
In order to address the specific needs of each of the scientific research activities mentioned hereafter, variations of the CR methodology are being developed. For manual operating systems, a microscope slide-based Cell Retainer, containing 7,000 picowells, has been developed. On the other hand, in order to address heavy load measurements, as required for drug discovery, 24- and 96-wells microplate-based CR (MCR) has been developed. In the latter case, each of the 96 "large wells" contains 70,000 picowells. The external shape and dimensions of the MCR are exactly the same as in the commonly used microplates, in order to allow for an easy and fast implementation of the MCR in the existing high-throughput/ high-content screening monitors.
Cutting edge material technology and MEMS (micro-electro-mechanical systems) processing techniques are implemented for the development of advanced CRs. These techniques include Glass microetching, Metal electro-plating, PDMS (polydimethyl siloxane) silicone soft-lithography (enabling rapid CR molding and replicating as well as oxygen permeability through the CR structure), Hydrogel formation (calcium activated, invisible in cell media), Porous materials (enabling a better cell settlement in the CR by applying sub-pressure through the CR, plus the unique capability of reagent diffusion and cell treatment via the CR basis), Teflon embossing (Teflon is hydrophobic and transparent in cell media due to their refractive index match), Sol-gel solidification and UV adhesive polymerization at room temperature.
Combining the optical characteristics with electrochemical sensing/stimulation of cells is being achieved by padding the CR with arrays of individually controlled, in-PW incorporated transparent micro-electrodes. The development of these electrodes, as well as their associated electronic communication, amplification and calibration, represent cutting edge technological capabilities.
Optical data acquisition
Optical data acquisition
Optical data acquisition is based on image analysis and fast signal analysis, utilizing the unique optical features of the CR microlens array. The CR optical features exclusively enable fast signal analysis – a presently nonexistent capability: The focal image of the microlens in the camera plane is used to relate a cluster of pixels to a respective microlens/microwell, thus creating an array of "clustered-detectors-per-picowell-per-cellâ€, in which each cluster detector probes only the light signal gathered from its corresponding picowell. Thus, with the CR, data can be rapidly and simultaneously acquired from the observed cells, bypassing complicated image analysis. Software packages have been developed to automatically govern, acquire, analyze, compare and archive all experimental data. This project has been initiated by the Jerome Schottenstein Center, and proceeds in collaboration with Molecular Devices Corporation, Sunnyvale, CA – the developers of MetaMorph - the world leader in image analysis software.
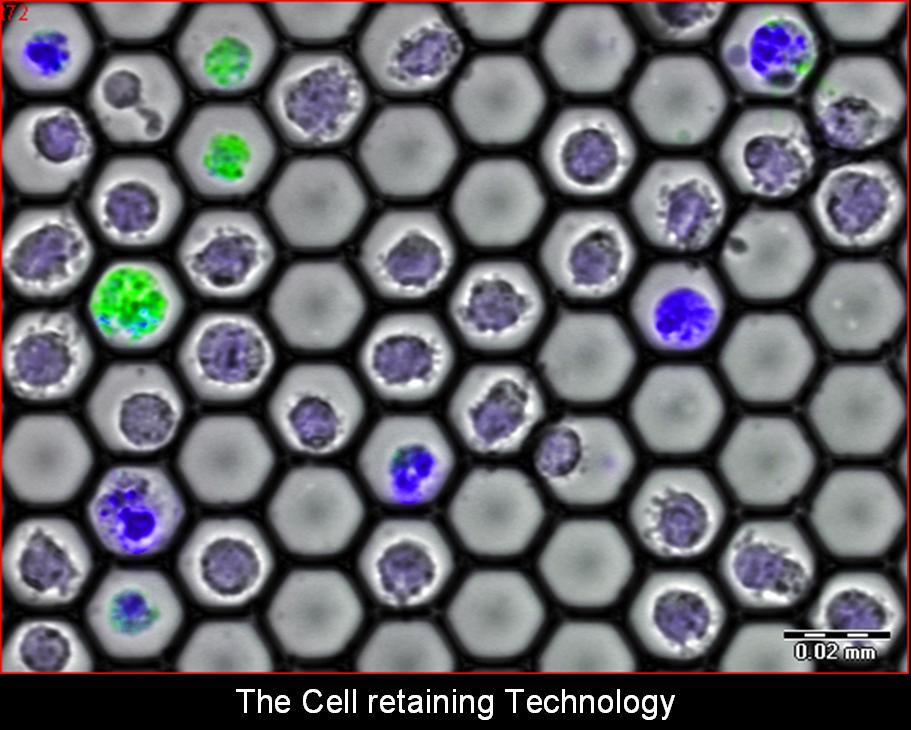
Cell-Retaining (CR) methodology and optical measurements
The essential features of the CR are being developed by implementing the concept of a closely packed 2-Dimensional array of high-quality transparent negative micro-lenses, where each concave micro-lens functions as a picoliter-well (PW) designed to hold a single cell without tethering. Various PW sizes and geometries are being designed to best fit specific individual cell or cell-cluster sizes, ranging from 15µm (for single lymphocytes) through 100µm (for cell-to-cell interaction/communication studies on small groups of cells) to 250µm (for cell islets).
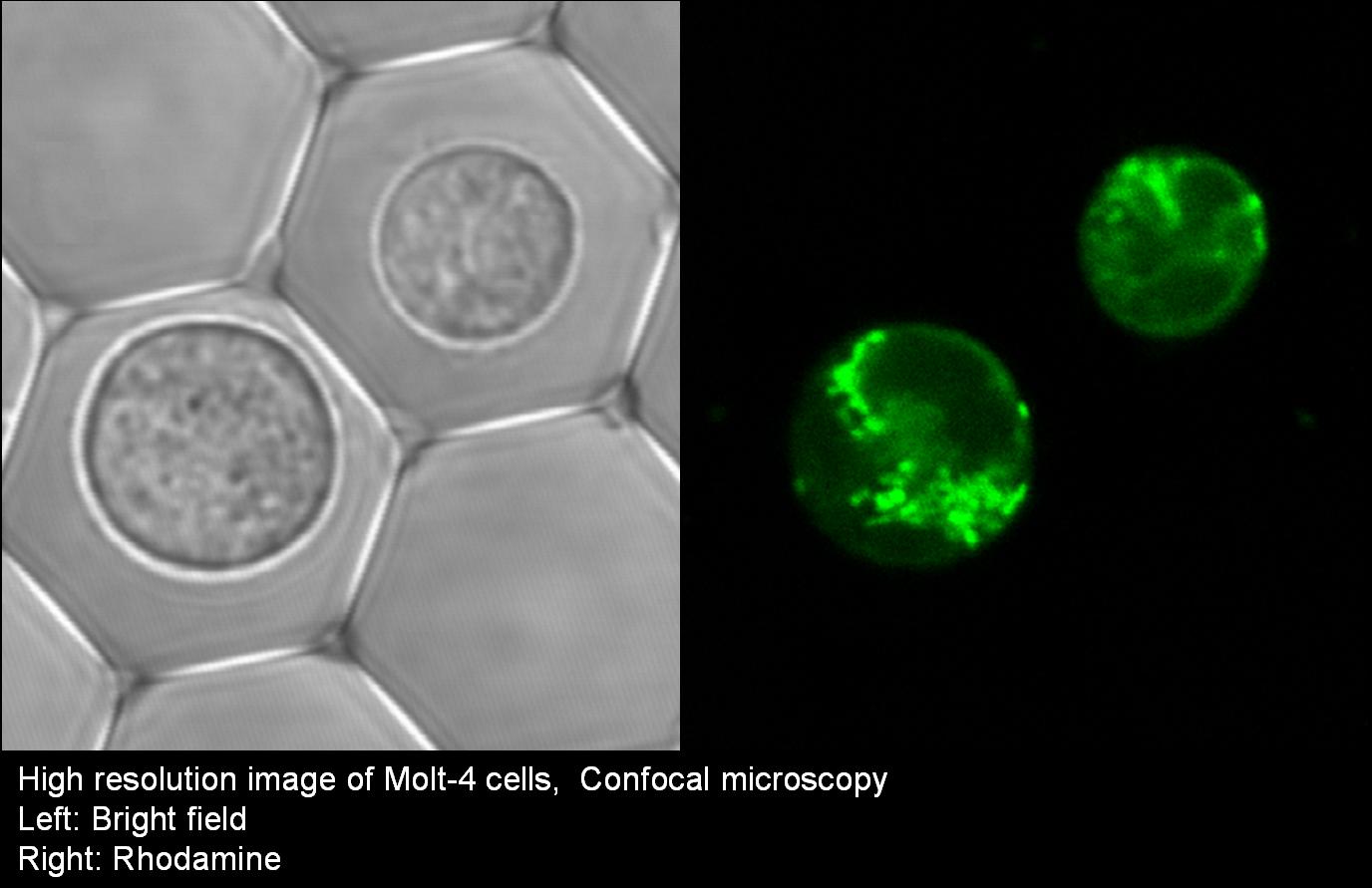
The Cell Retainers are designed to allow continuous cell treatments and bio-manipulations (staining, rinsing, media exchange, etc.) during sample observations via various types of microscopy: upright, inverted, confocal, multi-photon excitation, etc. The cells’ multi-parametric observations include transmitted light and various fluorescence measurements: FP (Fluorescence Polarization), FRET (Fluorescence Resonance Energy Transfer), FLIM (Fluorescence Lifetime Imaging), FRAP (Fluorescence Recovery After Photo-bleaching), FLIP (Fluorescence Loss in Photobleaching), FLAP (Fluorescence Localization After Photobleaching) and TIRF (Total Internal Reflection Fluorescence microscopy).